
If you work in medical device regulation, you’ve likely felt the frustration: you know the FDA has approved similar devices, issued guidance, and published summaries — and yet you spend hours, sometimes days, trying to find the information you need.
That’s because while the FDA’s playbook is technically public, it was never designed to be searchable, strategic, or user-friendly.
At Agent Astro, we’re solving that problem — by turning thousands of static documents into an intelligent, searchable knowledge base for regulatory professionals. Here’s how we do it — and why it matters more than ever.
The FDA Shares Almost Everything — That’s the Opportunity
The U.S. Food and Drug Administration publishes an extraordinary amount of information related to device approvals and regulatory processes. This includes:
In short: the regulatory playbook is out in the open. Every decision, precedent, and pattern is available — in theory.
The Problem: It’s Buried in PDFs and Inconsistent Formats
While the information is public, it’s also fragmented, inconsistently structured, and scattered across multiple databases. Searching for relevant data typically means:
Even experienced regulatory professionals often start from scratch — not because they don’t know what to do, but because they don’t have tools that make the public data usable.
The Insight: The Answers Are Already Out There — Hidden in Plain Sight
Strong submissions don’t need to reinvent the wheel. The language, structure, and rationale that succeeded in the past can inform smarter strategies today.
That’s the core idea behind Agent Astro:
If the regulatory playbook is already written, let’s make it accessible — and actionable.
Every 510(k) cleared, every De Novo granted, every PMA approved holds insights about what works:
But until now, there was no fast, reliable way to mine those insights — at scale.
From Data Chaos to Clarity: How Agent Astro Makes the FDA Playbook Usable
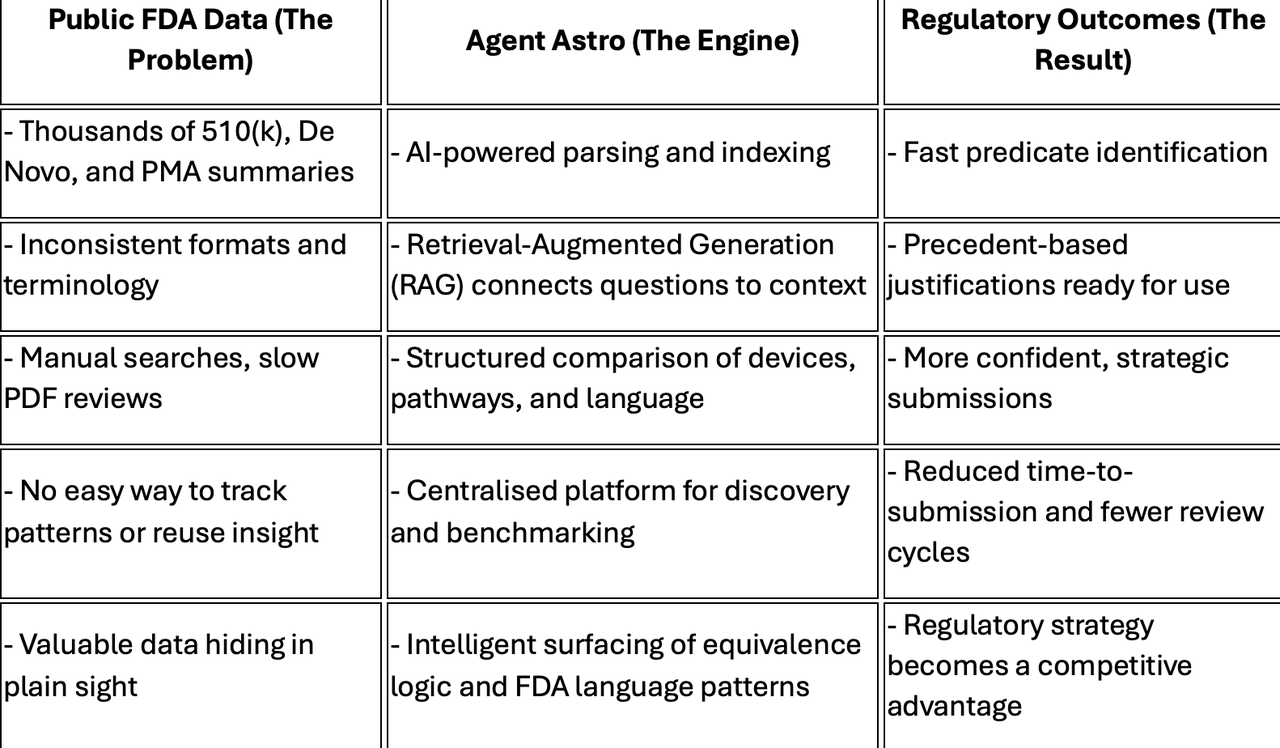
How Agent Astro Makes the FDA Playbook Searchable
Here’s how we turn scattered regulatory documents into strategic intelligence:
🔎 AI-Powered Indexing of FDA Submissions
We parse and structure thousands of summaries, mapping key elements like device class, intended use, performance testing, and equivalence language — turning unstructured PDFs into searchable data.
🧠 Retrieval-Augmented Generation (RAG)
Agent Astro uses RAG to pair verified regulatory content with natural language prompts. That means you can ask a question and get not just an answer — but a citation from a real FDA decision to back it up.
🧩 Predicate Discovery, Reimagined
Our system cross-references devices by characteristics, not just keywords — allowing you to discover predicate options and similar devices based on real-world relevance, not guesswork.
🗂 Submission Intelligence, On Demand
We surface submission structure patterns: how testing was presented, how equivalence was justified, what performance benchmarks were used — so you can model success, not chase it.
What Happens When the Playbook Becomes Searchable?
When regulatory teams can access this information easily, the shift is immediate and dramatic:
This doesn’t just save time. It builds confidence. It reduces review cycles. And it helps level the playing field for smaller companies competing with resource-heavy giants.
Regulatory Intelligence, Finally Within Reach
We didn’t build Agent Astro to replace regulatory professionals — we built it to empower them. You still lead the strategy, the judgement calls, and the submission. We just give you faster, better access to the data that matters most.
The FDA’s knowledge is written in public. You just need the right lens to read it.